Which of the following carbocations is likely to rearrange? This intriguing question lies at the heart of carbocation chemistry, a fascinating field that explores the behavior of these highly reactive intermediates. Carbocations, with their positively charged carbon atoms, undergo a myriad of rearrangements, dictated by their stability and the intricate interplay of electronic and steric factors.
Delving into the intricacies of carbocation rearrangement, this article unveils the factors that govern their reactivity, providing a comprehensive understanding of these fundamental chemical transformations.
Carbocation stability plays a pivotal role in determining the likelihood of rearrangement. Stable carbocations, characterized by resonance stabilization or electron-withdrawing substituents, resist rearrangement. Conversely, unstable carbocations, lacking such stabilizing features, readily undergo rearrangement to attain a more stable configuration. Understanding the factors that influence carbocation stability is crucial for predicting their rearrangement behavior.
Carbocation Rearrangement Overview: Which Of The Following Carbocations Is Likely To Rearrange
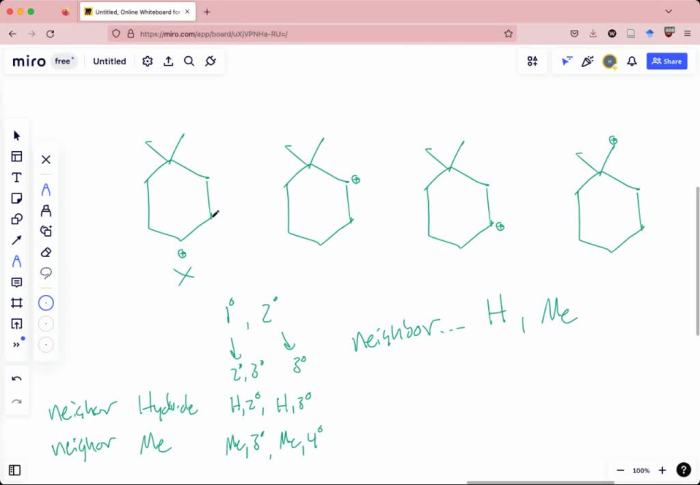
Carbocation rearrangements are chemical reactions in which a carbocation (a positively charged carbon atom) undergoes a structural change to form a more stable carbocation. These rearrangements are driven by the desire of the carbocation to achieve a lower energy state.
The factors that influence carbocation rearrangement include the stability of the starting and product carbocations, the nature of the substituents on the carbocation, and the reaction conditions.
Carbocation Stability
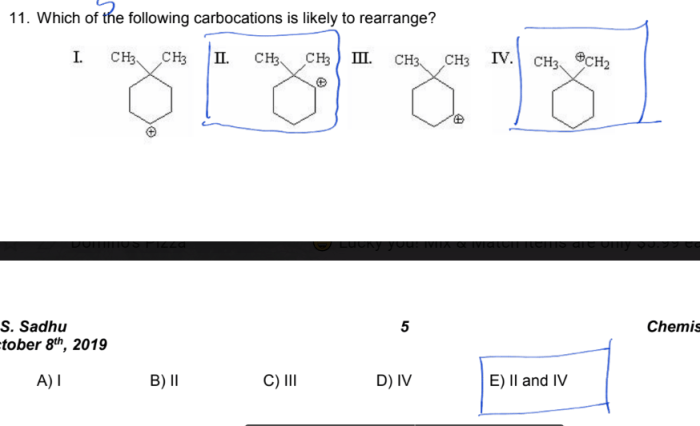
The stability of a carbocation is determined by the number of alkyl groups attached to the positively charged carbon atom. The more alkyl groups that are attached, the more stable the carbocation. This is because the alkyl groups donate electrons to the positively charged carbon atom, which helps to stabilize the charge.
Some examples of stable carbocations include the tertiary carbocation (CH 3) 3C +and the secondary carbocation (CH 3) 2CH +. Some examples of unstable carbocations include the primary carbocation CH 3CH 2+and the methyl carbocation CH 3+.
The relationship between carbocation stability and rearrangement is that the more stable the starting carbocation, the less likely it is to rearrange. This is because the starting carbocation is already in a relatively low energy state, and it would require a significant amount of energy to rearrange to a more stable carbocation.
Carbocation Rearrangement Mechanisms
There are two main mechanisms of carbocation rearrangement: 1,2-shifts and 1,3-shifts. In a 1,2-shift, a hydrogen atom or an alkyl group moves from one carbon atom to an adjacent carbon atom. In a 1,3-shift, a hydrogen atom or an alkyl group moves from one carbon atom to a carbon atom that is two atoms away.
Some examples of 1,2-shifts include the rearrangement of the tertiary carbocation (CH 3) 3C +to the secondary carbocation (CH 3) 2CH +and the rearrangement of the secondary carbocation (CH 3) 2CH +to the primary carbocation CH 3CH 2+.
Some examples of 1,3-shifts include the rearrangement of the tertiary carbocation (CH 3) 3C +to the primary carbocation CH 3CH 2CH 2+and the rearrangement of the secondary carbocation (CH 3) 2CH +to the methyl carbocation CH 3+.
The stereochemistry of carbocation rearrangement depends on the mechanism of the rearrangement. In a 1,2-shift, the migrating group moves with its own pair of electrons, and the stereochemistry of the starting carbocation is retained in the product carbocation. In a 1,3-shift, the migrating group moves without its own pair of electrons, and the stereochemistry of the starting carbocation is inverted in the product carbocation.
Predicting Carbocation Rearrangement
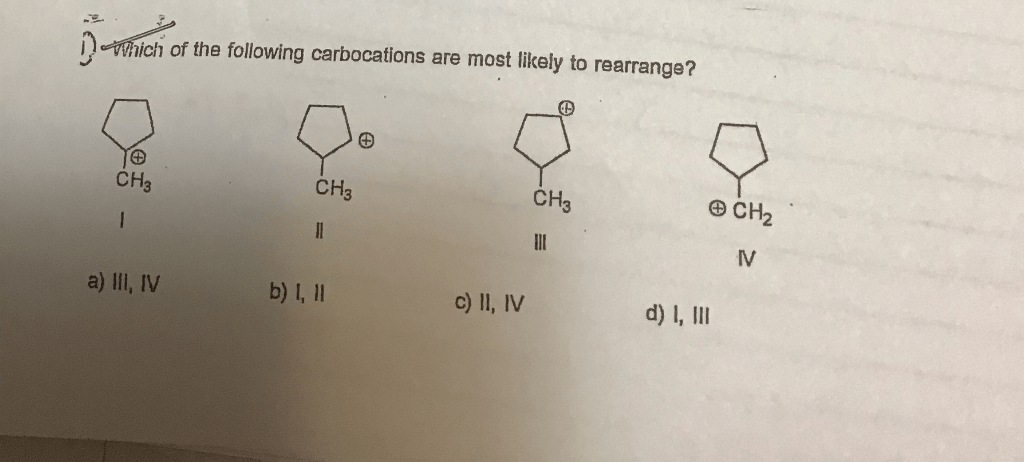
The products of carbocation rearrangement can be predicted by using the following guidelines:
- The starting carbocation will rearrange to the most stable carbocation possible.
- The rearrangement will proceed through the lowest energy pathway.
- The stereochemistry of the starting carbocation will be retained or inverted, depending on the mechanism of the rearrangement.
Resonance structures can be used to help predict the products of carbocation rearrangement. Resonance structures are different Lewis structures that represent the same molecule. The most stable resonance structure is the one with the lowest energy. The products of carbocation rearrangement will be the carbocations that are represented by the most stable resonance structures.
Examples of Carbocation Rearrangement
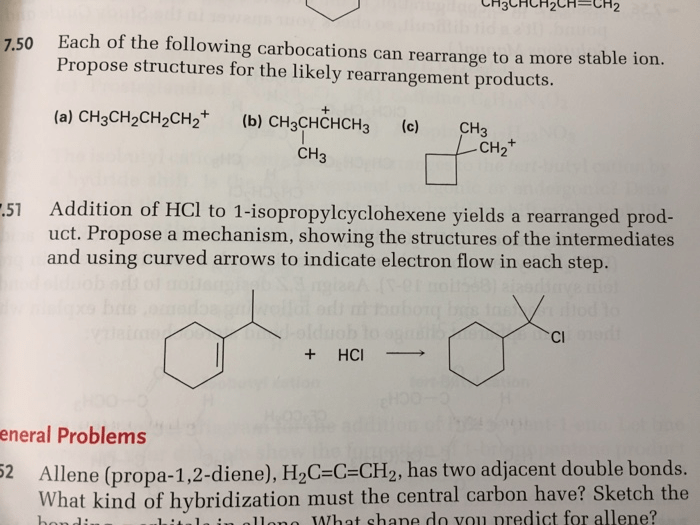
Carbocation rearrangements are common in organic chemistry. Some examples of carbocation rearrangement reactions include:
- The Friedel-Crafts alkylation reaction
- The Diels-Alder reaction
- The Claisen rearrangement
- The Wagner-Meerwein rearrangement
These reactions are used to synthesize a wide variety of organic compounds.
Question Bank
What is carbocation rearrangement?
Carbocation rearrangement is a chemical reaction in which a carbocation undergoes a structural change to form a more stable carbocation.
What factors influence carbocation rearrangement?
Carbocation stability, resonance effects, and steric interactions are the primary factors that influence carbocation rearrangement.
How can we predict the products of carbocation rearrangement reactions?
By considering the stability of the carbocation intermediates and the potential resonance structures, we can predict the products of carbocation rearrangement reactions.